The Team: Lingbang Zhu, Jeshurun Luke, Dr. Yi-Xiang Liu
Past members: Yu Liu, Ming-Guang Hu, David Grimes, Matthew Nichols, Mark Babin
Details of this beautiful apparatus shown in the above picture can be found in this PCCP perspective.
Chemical reactions can be surprisingly efficient at cold (1 mK) and ultracold temperatures (1 µK) due to the wave nature of atoms and molecules. At these temperatures, non-classical effects such as wavefunction delocalization and tunneling through barriers, can dominate the reaction rate. A mere change of the quantum statistics of reagents at an energy typically near 10-8 kcal/mol, can further alter the reaction rate by a factor of 10 to 100.
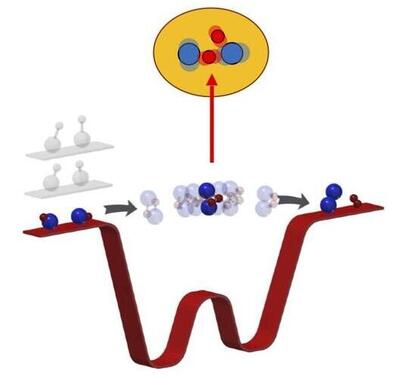
Studies of reaction kinetics explore elementary reaction steps. We are embarking on a project study the unique 4-center reaction of 2 KRb -> K2+Rb2 at ultracold temperatures. In KRb, the 4-center reaction which begins at 1 µK and ends with 10K exothermic energy has to transition through a very deep potential well (4000K). Such an energy mismatch between the entrance to exit channels and the intermediate transition suggests that the intermediate complex may be long-lived. Classically, one expects most reactant trajectories with a wide range of initial velocities and angles would take a long time to find their way out of such a deep potential well. This reaction, which is slow and highly unlikely to proceed classically, may proceed efficiently and prove quantum mechanics is efficient at exploring a vast range of reaction phase space. Which scenario is true awaits experimental result.
We combine atomic, molecular, and optical (AMO) physics techniques to prespare ultracold reactants and physical chemistry techniques to detect reaction products through ionization by either REMPI or velocity-map imaging.
- (2018) After 4 years of hard work, we successfully combined ultracold molecule creation with velocity map imaging ion detection to reveal the KRb+KRb reaction!
- (2019) Observing and steering ultracold reactions at 500nK! Check out our papers in Science where we directly detect reaction products and a long-lived intermediate complex at this record-low temperature and in Nature Physics where we investigate the properties of the complex including its interaction with 1064 nm light.
|
|
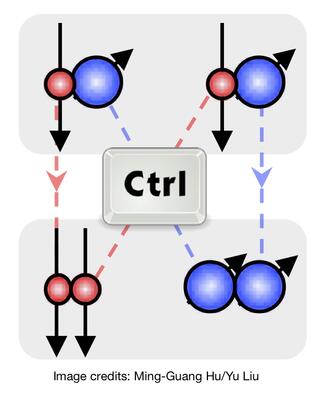
- (2020) Establishing nuclear spin conservation in ultracold KRb bimolecular reaction and Controling rotation state (parity) of ultracold reaction products. Check out our paper here.
|
-
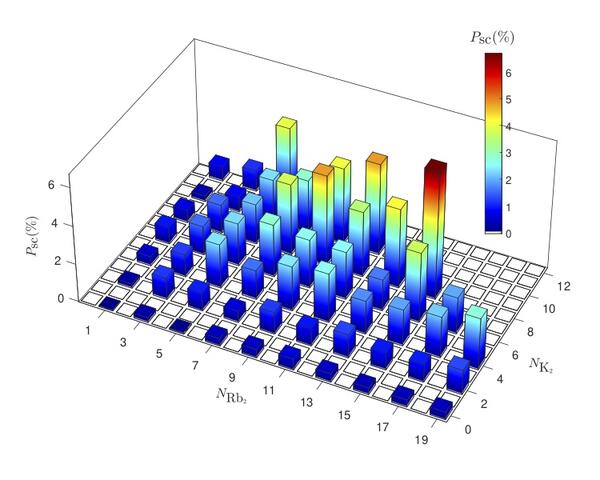
(2020) Most recently, we developed a method for coincident detections of reaction products to allow a full quantum-state resolved study of the ultracold KRb reaction. This allowed us to precisely examine the reaction dynamics which was assumed to be statistical in nature. Read more about it here.
We are continuing to investigate checmial reactions (molecule-molecule and atom-molecule reactions) at the deepest level. Next, we hope to study entanglement and quantum interference in ultracold chemical reactions.