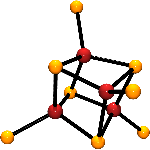
Carbon monoxide dehydrogenases (CODH) catalyze the reaction CO + H2O → CO2 + 2H+ + 2e-. Bifunctional enzymes also catalyze the formation of acetylcoenzyme A from carbon monoxide, a methyl group supplied by a corrinoid protein, and coenzyme A. The acetylcoenzyme A synthase activity occurs at the A-cluster. The CO/CO2 interconversion reaction occurs at the C-cluster. Both clusters have been revealed by protein crystallography. The A-cluster is a bridged assembly with minimal formulation [Fe4S4]-(µ2-Scys)-[Ni((µ2-Scys)2Gly)Ni]. It has been demonstrated that the dinickel content is essential for activity. The C-cluster contains a NiFe4(µ3-S)4 core with distorted tetrahedral iron sites, among which is an Fe atom that is positioned exo to the cubanoid NiFe3S4 portion of the core.

Both clusters represent substantial challenges in the synthesis of the weak-field heterometal clusters that are analogues of the native clusters in biology. Currently, we are engaged in the problem of constructing analogues of both clusters.  We have prepared the double bridged Ni2(µ2-SR)2 rhomb and explored the methodology to achieve the Fe-(µ2-SR)-Ni II structure pertinent to the A-Cluster. Cluster motifs that converge on the C-cluster have also been isolated in our laboratories, where the tetrahedral Ni II site in the cubane-type cluster [(Ph3P)NiFe3S4(LS3)]2- can be converted to a planar diamagnetic configuration by two different methods. We are currently developing a means to incorporate bridging atoms that promote binding of the exo Fe atom to form the full NiFeS5 core.
We have prepared the double bridged Ni2(µ2-SR)2 rhomb and explored the methodology to achieve the Fe-(µ2-SR)-Ni II structure pertinent to the A-Cluster. Cluster motifs that converge on the C-cluster have also been isolated in our laboratories, where the tetrahedral Ni II site in the cubane-type cluster [(Ph3P)NiFe3S4(LS3)]2- can be converted to a planar diamagnetic configuration by two different methods. We are currently developing a means to incorporate bridging atoms that promote binding of the exo Fe atom to form the full NiFeS5 core.