The nitrogenase enzyme catalyzes the key reductive step of dinitrogen to ammonia in the global biological nitrogen. The Mo-dependent nitrogenase contains two component proteins that are designated the Fe protein and the MoFe protein. The Fe protein acts as an agent for electron transfer by delivering the necessary reducing equivalents to the MoFe protein, the location of substrate activation and reduction. The enzyme relies on three high nuclearity metal-sulfur complexes to carry out this complicated process: an Fe4S4 cubane unit in the Fe protein; and a P-cluster and iron-molybdenum cofactor (FeMo-cofactor) in the MoFe protein. The macromolecular structure determination of the MoFe protein has revealed a [MoFe7S9] cluster composed of two sulfur-voided [M4S3] cuboidal fragments linked by three µ2-sulfur bridges. Higher-resolution (2.0-Å) protein crystal structures have subsequently revealed the P-cluster to be a symmetric [Fe8S7] structure comprising, in the native state (PN), two [Fe4S3] cuboidal halves, vertex-fused through a µ6-sulfide and further interconnected by two µ2-cysteinate bridges. All these cluster structures are remarkable from a synthetic perspective, and we are exploring new chemistry to better understand the properties of these cluster units.
The nitrogenase enzyme catalyzes the key reductive step of dinitrogen to ammonia in the global biological nitrogen. The Mo-dependent nitrogenase contains two component proteins that are designated the Fe protein and the MoFe protein. The Fe protein acts as an agent for electron transfer by delivering the necessary reducing equivalents to the MoFe protein, the location of substrate activation and reduction. The enzyme relies on three high nuclearity metal-sulfur complexes to carry out this complicated process: an Fe4S4 cubane unit in the Fe protein; and a P-cluster and iron-molybdenum cofactor (FeMo-cofactor) in the MoFe protein. The macromolecular structure determination of the MoFe protein has revealed a [MoFe7S9] cluster composed of two sulfur-voided [M4S3] cuboidal fragments linked by three µ2-sulfur bridges. Higher-resolution (2.0-Å) protein crystal structures have subsequently revealed the P-cluster to be a symmetric [Fe8S7] structure comprising, in the native state (PN), two [Fe4S3] cuboidal halves, vertex-fused through a µ6-sulfide and further interconnected by two µ2-cysteinate bridges. All these cluster structures are remarkable from a synthetic perspective, and we are exploring new chemistry to better understand the properties of these cluster units.
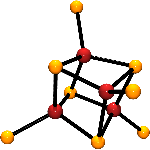
Pursuant to these goals, we have demonstrated that the reduction of phosphine-ligated [MFe3S4] cubanes (M = Fe, Mo, V) yield edge-bridged double-cubane [M2Fe6S8] clusters that approach the core composition and overall extended geometry of symmetrized versions of the FeMo-cofactor. In an effort to realize clusters with cofactor-exact 8:9 metal/sulfide stoichiometries, these double-cubane products can be treated with sulfide reagents to incorporate sulfur into the core. The rearranged cluster core that is generated contains the desired [M8S9] composition. Despite the constitutional similarity, the resultant clusters do not adopt cofactor-like cores but instead bear a striking near-congruence to the native form of the nitrogenase PN-cluster (if bridging µ2-sulfides are substituted for the µ2-cysteinates in the biological system). We are currently investigating how the edge-bridged double cubane is transformed into the PN-cluster arrangement, and aim to use this information to incorporate a heteroatom into the assembly to form a structural analogue to the FeMo-cofactor.
Copyright © 2024 The President and Fellows of Harvard College | Accessibility | Digital Accessibility | Report Copyright Infringement