Xingyi Deng and Thomas A. Baker
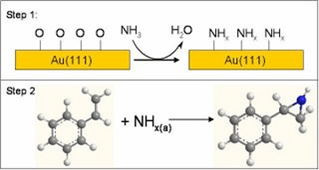
Gold-based heterogeneous catalysts have been shown to have a surprising potential as selective catalysts for redox processes. Such catalysts have been investigated for catalytic alcohol oxidation, the direct synthesis of hydrogen peroxide, low temperature CO oxidation, and olefin epoxidation. These systems are of interest because the low temperature at which they operate and their high selectivity have potential for substantial positive impact on the environment and economy. For example, low-temperature CO oxidation using gold has the potential to overcome the cold-startup problem in automotive pollution control, since the Pt- or Pd-based catalyst currently used are inactive below 200C. Hence, a substantial amount of effort has been directed to further improve the performance of heterogeneous gold catalysts and to understand the origin of their catalytic activity.
In spite of the rapidly increasing number of studies of Au catalysis, the entire focus has been on exploring known oxidation reactions. Because of the importance in organic synthesis as building blocks and potential in pharmaceuticals, we have investigated the possibility of using Au to promote aziridine synthesis. Previously, we have shown that a structurally well-defined single crystal surface of gold, Au(111), promotes oxidation reactions that also occur on high-surface area catalysts at higher pressures once oxygen has been adsorbed on the surface (19). This previous work clearly establishes that Au(111) is a good model for understanding molecular-level details of Au-based oxidation catalysis. Hence, reactions that occur on Au(111) should serve as a guide for the type of reactions that may be induced by heterogeneous gold catalysts.

